Table of Contents
ToggleNCERT Solutions Class 10 Science Chapter 1 (Chemical Reactions and Equations)
NCERT Solutions for Class 10 Science Chapter 1 Intext Questions
NCERT Solutions Class 10 Science Chapter 1 (Chemical Reactions and Equations)
Page Number: 6
Question 1
Why should a magnesium ribbon be cleaned before burning in air ?
Answer:
Magnesium gets covered with a layer of magnesium oxide when kept in air for a long time. This layer hinders the burning of magnesium. Hence, it is to be cleaned before burning.
Question 2
Write the balanced equation for the following chemical reactions.
(i) Hydrogen + Chlorine → Hydrogen chloride
(ii) Barium chloride + Aluminium sulphate → Barium sulphate + Aluminium chloride
(iii) Sodium + Water → Sodium hydroxide + Hydrogen
Answer:

Question 3
Write a balanced chemical equation with state symbols for the following reactions :
(i) Solutions of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and the solution of sodium chloride.
(ii) Sodium hydroxide solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium chloride solution and water.
Answer:

NCERT Solutions Class 10 Science Chapter 1 (Chemical Reactions and Equations)
Page Number: 10
Question 1
A solution of a substance ‘X’ is used for white washing.
(i) Name the substance ‘X’ and write its formula.
(ii) Write the reaction of the substance ‘X’ named in (i) above with water.
Answer:

Question 2
Why is the amount of gas collected in one of the test tubes in text book Activity 1.7 (i.e., electrolysis of water) double of the amount collected in the other? Name this gas.
Answer:

NCERT Solutions Class 10 Science Chapter 1 (Chemical Reactions and Equations)
Page Number: 13
Question 1
Why does the colour of copper sulphate solution change when an iron nail is dipped in it ?
Answer:

Question 2
Give an example of a double displacement reaction other than the one given in Activity 1.10
Answer:

Question 3
Identify the substances that are oxidised and the substances which are reduced in the following reactions.
(i) 4Na(s) + O2(g) → 2Na2O(s)
(ii) CuO (s) + H2(g) → Cu (s) + H2O(l)
Answer:

NCERT Solutions for Class 10 Science Chapter 1 Exercise Questions (Textbook Chapter End Questions)
NCERT Solutions Class 10 Science Chapter 1 (Chemical Reactions and Equations)
Question 1
Which of the statements about the reaction below are incorrect ?
2 PbO(s) + C(s) → 2Pb (s) + CO2(g)
(a) Lead is getting reduced.
(b) Carbon dioxide is getting oxidised.
(c) Carbon is getting oxidised.
(d) Lead oxide is getting reduced.
(i) (a) and (b)
(ii) (a) and (c)
(iii) (a), (b) and (c)
(iv) All
Answer:

Question 2
Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a
(a) combination reaction
(b) double displacement reaction
(c) decomposition reaction
(d) displacement reaction
Answer:

Question 3
What happens when dilute hydrochloric acid is added to iron filings ? Tick the correct answer :
(a) Hydrogen gas and iron chloride are produced.
(b) Chlorine gas and iron hydroxide are produced.
(c) No reaction takes place.
(d) Iron salt and water are produced.

Question 4
What is a balanced chemical equation ? Why should chemical equations be balanced ?
Answer:

Question 5
Translate the following statements into chemical equations and then balance them.
(a) Hydrogen gas combines with nitrogen to form ammonia.
(b) Hydrogen sulphide gas burns in air to give water and sulphur dioxide.
(c) Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
(d) Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
Answer:

Question 6
Balance the following chemical equations :
(a) HNO3 + Ca (OH)2 → Ca (NO3)2 + H2O
(b) NaOH + H2SO4 → Na2SO4 + H2O
(c) NaCl + AgNO3 → AgCl + NaNO3
(d) BaCl2 + H2SO4 → BaSO4 + HCl
Answer:
(a) 2HNO3 + Ca(OH)2 → Ca(NO3)2 + 2H2O
(b) 2NaOH + H2SO4 → Na2SO4 + 2H2O
(c) NaCl + AgNO3 → AgCl + NaNO3
(d) BaCl2 + H2SO4 → BaSO4 + 2HCl
Question 7
Write the balanced chemical equations for the following reactions :
(a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water
(b) Zinc + Silver nitrate → Zinc nitrate + Silver
(c) Aluminium + Copper chloride → Aluminium chloride + Copper
(d) Barium chloride + Potassium sulphate → Barium sulphate + Potassium chloride
Answer:
(a) Ca (OH)2 + CO2 → CaCO3 + H2O
(b) Zn + 2AgNO3 → Zn(NO3)2 + 2 Ag
(c) 2Al + 3 CuCl2 → 2AlCl3 + 3 Cu
(d) BaCl2 + K2SO4 → BaSO4 + 2KCl
Question 8
Write the balanced chemical equation for the following and identify the type of reaction in each case :
(a) Potassium bromide (aq) + Barium iodide (aq) → Potassium iodide (aq) + Barium
(b) Zinc carbonate(s) → Zinc oxide (s) + Carbon dioxide (g) bromide(s)
(c) Hydrogen (g) + Chloride (g) → Hydrogen chloride (g)
(d) Magnesium (s) + Hydrochloric acid (aq) → Magnesium chloride (aq) + Hydrogen (g)
Answer:
(a) 2KBr (aq) + Bal2(aq) → 2Kl(aq) + BaBr2(s)
Type : Double displacement reaction
(b) ZnCO3 (s) → ZnO (s) + CO2 (g)
Type : Decomposition reaction
(c) H2 (g) + Cl2 (g) → 2HCl(g)
Type : Combination reaction
(d) Mg (s) + 2HCl (aq) → MgCl2 (aq) + H2 (g)
Type : Displacement reaction
Question 9
What does one mean by exothermic and endothermic reactions ? Give examples.
Answer:

Question 10
Why is respiration considered an exothermic reaction ? Explain.
Answer:

Question 11
Why are decomposition reactions called the opposite of combination reactions? Write equations for these reactions.
Answer:

Question 12
Write one equation each for the decomposition reactions where energy is supplied in the form of heat, light or electricity.
Answer:

Question 13
What is the difference between displacement and double displacement reactions? Write equations for these reactions.
Answer:

Question 14
In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the reaction involved.
Answer:
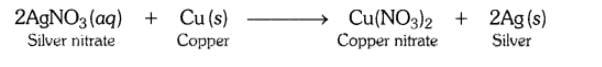
Question 15
What do you mean by a precipitation reaction ? Explain by giving examples.
Answer:
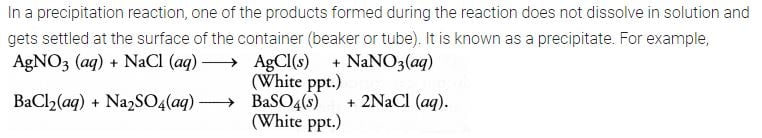
Question 16
Explain the following in terms of gain or loss of oxygen with two examples each:
(a) Oxidation and
(b) Reduction.
Answer:
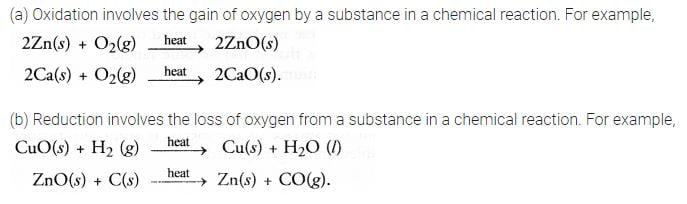
Question 17
A shiny brown coloured element ‘X’ on heating in air becomes black in colour. Name the element ‘X’ and the black coloured compound formed.
Answer:
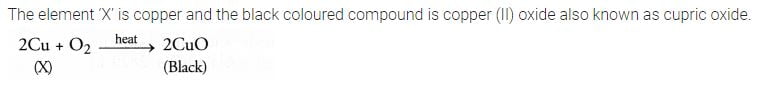
Question 18
Why do we apply paint on iron articles ?
Answer:
Paint does not allow iron articles to come in contact with air, water and saves iron articles from damage due to rusting.
Question 19
Oil and fat containing food items are flushed with nitrogen. Why ?
Answer:

Question 20
Explain the following terms with one example each
(a) Corrosion,
(b) Rancidity.
Answer:


NCERT Solutions Class 10 Science Chapter 1 Chemical Reactions and Equations Provided by Bhautik Study covers all the essential subjects in great detail to aid in students’ comprehension of the material. NCERT Solutions Class 10 Science Chapter 1 Chemical Reactions and Equations NCERT Solutions are required reading for students getting ready for their exams. You can review the entire syllabus and earn more marks by using the answers to all of the Chapter 1: Chemical Reactions and Equations Exercise Questions.
Let’s take a quick look at a list of themes and subtopics under NCERT Solutions Class 10 Science Chapter 1 Chemical Reactions and Equations before delving into the specifics of the NCERT Solutions Class 10 Science Chapter 1 Chemical Reactions and Equations:
1) Equations And Chemical Reactions
2) Chemical Formulas
3) Chemical Reaction Types
4) Have You Noticed How Oxidation Reactions Affect Your Daily Life? Y Life?
Characteristics of NCERT Solutions Class 10 Chapter 1 Science Pupils might receive extensive instruction in balancing various types of equations.
-With the help of the CBSE Class 10 Chemistry learning tools, discover how to write a balanced chemical equation and learn about chemical reactions.
-You can write different chemical equations correctly with the aid of NCERT Solution. aids in providing you with extensive practice answering questions of various levels of difficulty before the main exam.
-When completing homework assignments and getting ready for tests, our extensive collection of study materials serves as an ideal roadmap.
-Our specialists at Bhautikstudy.com help you learn chemistry by providing CBSE Class 10 Chemistry notes, MCQs, and NCERT solutions that follow the most recent syllabus.
By using the Bhautikstudy.com NCERT Solutions Learning App on your smartphone, you can access free conceptual videos and LIVE master classes. Learn the NCERT Solutions Class 10 Science Chapter 1 (Chemical Reactions and Equations) NCERT Book Solution in detail from knowledgeable science instructors.
Having a thorough understanding of the material and practicing will help you get 100% on the questions in this chapter. It’s now simple to learn the principles of chemistry in CBSE Class 10.
We hope that this comprehensive set of NCERT Solutions will be useful to you now that you have all the information you need to solve the Chemical Reactions NCERT Solutions Science Chapter 1 problems. Bhautikstudy.com offers free access to NCERT Books, CBSE Syllabus, CBSE Sample Papers, and RD Sharma Solutions for students.
Excellent study materials are required for students studying in Class 10 CBSE Chemistry Chapter 1 Chemical Reactions and Equations, according to NCERT Solutions Class 10 Science Chapter 1. Subject matter specialists at Bhautik Study have created these NCERT Solutions in accordance with the most recent CBSE Syllabus. It is crucial that students use NCERT Solutions Class 10 Science Chapter 1 to assist them learn how to solve and study so they can become familiar with the kinds of questions that are posed in the chapter as well as chemical reactions and equations.
The key topics covered in Chapter 1 of NCERT Solutions Class 10 Science Chapter 1 are creating chemical equations and writing and balancing equations. Additionally, students study the fundamentals of chemical reactions in this chapter, along with their various varieties and the practical applications of chemical oxidation reactions. An summary of the chapter’s key ideas is given in the NCERT Solutions Class 10 Science Chapter 1, which also helps students become knowledgeable about crucial subjects like creating and balancing chemical equations.
There are about four problems from Science Chapter 1 of the Class 10 NCERT Solutions each year, and they carry a good weight. The majority of the questions in this chapter are practice-based questions.
In our daily lives, chemical reactions are significant phenomena. Numerous chemical reactions occur in daily life, such as the rusting of iron, the curdling of milk, respiration, digestion, and development. Students need to practise using this NCERT Solution Class 10 Science Chapter 1 in order to do well on the CBSE Class 10 test.