Class-12 Ch-12 Atoms

About Course
Class-12 PHYSICS
Atoms class 12 notes
Ch-12 Atoms
Topic Covered
–Latest CBSE syllabus 2023-2024
-Introduction, J.J Thomson’s, Rutherford Experiment
-Distance of Closest Approach
-Energy of Orbiting Electron
-Bohr’s postulate
-Expression for radius of nth possible orbit,
– Velocity and Energy of electron in his orbit
– Hydrogen Line Spectra
-All important CBSE Numerical
Introduction
In atoms class 12 notes, we will study various atomic models. Initially, J.J. Thomson proposed an atomic model in which he thought of as electrons embedded in between protons. In 1911, his student Earnest Rutherford proposed a nuclear model, on the basis of a scattering experiment. In spite of strong experimental evidence, Rutherford’s model of the atom was rejected on the ground of the classical theory of electromagnetism.
So in order to rectify the shortcomings of Rutherford’s model, in 1913, Niels Bohr combined the classical and early quantum concepts of Einstein and Plank to explain the stability of an atom.
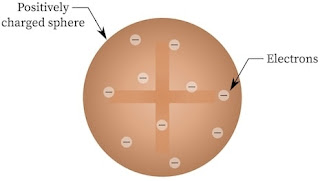
Thomson Model
According to Thomson, “An atom consists of positively charged matter, into which negatively charged particles are embedded randomly”. But this model did not last long as it could not explain the observations of Rutherford’s alpha-particle scattering experiment.
| Thomson atomic model |
Alpha-Particle Scattering
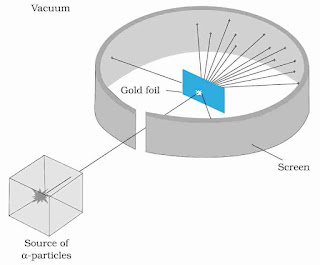
In 1911, Rutherford, along with his assistants, H. Geiger and E. Marsden, performed the Alpha Particle scattering experiment, which led to the birth of the ‘nuclear model of an atom’ it is a part of Atoms class-12 notes.
They took a thin gold foil having a thickness of 2.1×10-7 m and placed it in the center of a rotatable detector made of zinc sulfide and a microscope.
Next, they aimed a 5.5 MeV alpha particle beam at the foil using a radioactive source. These alpha particles were collimated as they travelled through lead bricks. The brief flashes on the screen allowed one to study the scattering of these alpha particles when they struck the foil. The outcomes of this experiment will be crucial in understanding atoms class 12 notes, as Rutherford and his colleagues anticipated learning more about the structure of the atom from them.
Observations
Here is what they found:
- Most of the alpha particles passed through the foil without suffering any collisions
- Around 0.14% of the incident alpha particles scattered by more than 1o
- Around 1 in 8000 alpha particles deflected by more than 90o
Rutherford’s Nuclear Model
Rutherford put forth his nuclear model of the atom in 1912. It is also referred to as Rutherford’s planetary model of atoms, and it is crucial to understanding of atoms class 12 notes. Salient features of Rutherford’s atom model are as follows :
Every atom consists of a tiny central core, named nucleus, in which the entire positive charge and the almost whole mass of the atom are concentrated. The size of the nucleus is typically 10-4 times the size of an atom.
- Most of an atom is empty space.
- In free space around the nucleus, electrons would be moving in orbits just as the planets do around the sun. The centripetal force needed for the orbital motion of electrons is provided by electrostatic attractive forced experience by electrons due to a positively charged nucleus.
- An atom as a whole is electrically neutral. Thus, the total positive charge of the nucleus is exactly equal to the total negative charge of all the electrons orbiting in an atom.
| Rutherford’s Atom Model |
Bohr Model of the Hydrogen Atom
It was Niels Bohr (1885-1962) who used the concept of quantized energy and explained the model of a hydrogen Atoms in 1913 were significant in the atoms class 12 notes. Bohr developed a theory consisting of three postulates by fusing ideas from early quantum theory with classical thought. These postulates are:
- Postulate I: An electron in an atom could revolve in certain stable orbits without emitting radiant energy. Each atom has certain definite stable orbits. Electrons can exist in these orbits. Each possible orbit has definite total energy. These stable orbits are called the stationary states of the atom.
- Postulate II: An electron can revolve around the nucleus in an atom only in those stable orbits whose angular momentum is the integral multiple of h/2π (where h is Planck’s constant). Therefore, the angular momentum (L) of the orbiting electron is quantized.
- Postulate III: An electron can make a transition from its stable orbit to another lower stable orbit play an important role in atoms class 12 notes. While doing so, a photon is emitted whose energy is equal to the energy difference between the initial and final states. Therefore, the energy of photon is given by,hυ = Ei – Efwhere Ei and Ef are the energies of the initial and final states.
Ground State and the Excited States
The lowest energy level of an atom is called the “ground state” and higher levels are called “excited states” play an important role in atoms class 12 notes. The H-atom has the lowest energy in the state for the principal quantum number n = 1. and all other states (i.e, for n = 2, 3, 4…) are excited states. Thus E2, E3, E4 …are called the first, the second, and the third …excited states respectively it play an important role in atoms class 12 notes.
Ionisation Energy and Ionisation Potential
“Ionisation energy” is the bare minimum of energy required to ionise an atom. “Ionisation potential” refers to the potential difference that must be created in order for an electron to accelerate and gain this much energy. As a result, the ground state ionisation energy of a H atom is 13.6 eV, and the ionisation potential is 13.6 V.
Binding Energy
The least amount of energy required for a system’s constituents to be separated over a considerable distance is known as the binding energy. This might alternatively be described as the energy released during the process of constructing the system’s components from infinity. The ground state binding energy of an H-atom is 13.6 eV, the same energy required for ionisation.
Excitation Energy and Excitation Potential
The “excitation energy” of an excited state is the amount of energy required to get an atom from its ground state to that excited state. The “excitation potential” is the potential that an electron should be propelled through in order to obtain this energy.
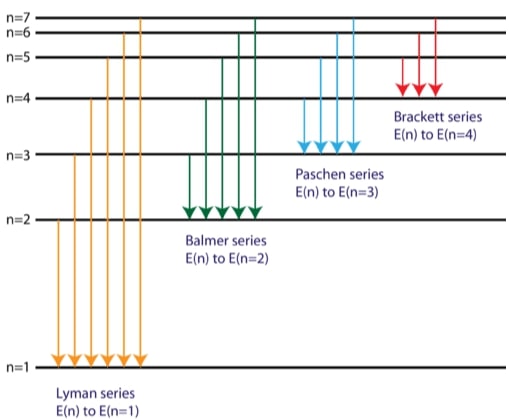
The Line Spectra of the Hydrogen Atom
The spectral lines of the hydrogen atomic emission spectrum in atoms class 12 notes are explained by Bohr’s model. The atom’s energy doesn’t change even though its electron stays in the ground state. The electron travels from the ground state orbit to a farther-off excited state orbit when the atom absorbs one or more quanta of energy. The energy released by the atom when it returns to a lower energy state is equal to the difference in energy between the two orbits.
Bohr was able to determine the energies that the hydrogen electron would have at each of its permitted energy levels based on the wavelengths of the spectral lines. He subsequently demonstrated through mathematics which energy level transitions were associated with the spectral lines in the atomic emission spectrum it play an important role in atoms class 12 notes.
R = 1.01 x 107 m-1 = Rydberg constant
He found that the four visible spectral lines corresponded to transitions from higher energy levels down to the second energy level (n = 2). This is called the Balmer series. Transitions ending in the ground state (n = 1) are called the Lyman series, but the energies released are so large that the spectral lines are all in the ultraviolet region of the spectrum. Because the energies are too low, the transitions known as the Paschen and Bracket series produce spectral lines in the infrared spectrum it play an important role in atoms class 12 notes.
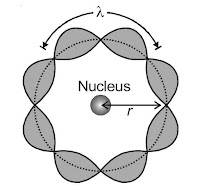
de-Broglie’s Explanation of Bohr’s Second Postulate
Assuming that an electron is a particle wave, de-Broglie provided an explanation for the second postulate of Bohr’s atomic model for atoms class 12 notes. As a result, in resonance circumstances, it ought to produce standing waves.
According to de-Broglie, for an electron moving in nth circular orbit of radius r,
2πr = nλ n = 1, 2, 3, …..
i.e., the circumference of the orbit should be an integral multiple of the de-Broglie wavelength of an electron moving in nth orbit. As we know that de-Broglie wavelength it play an important role in atoms class 12 notes.
Summary of atoms class 12 notes
Impact parameter:
Perpendicular distance of initial velocity vector of α-particles from the center of the nucleus.
Distance of closest approach: Distance of a point from the nucleus at which α-particle is nearest to the center of the nucleus.
- Bohr radius: The first orbit of a hydrogen atom is called the Bohr radius.
- Ground state: Lowest state of an atom, called the ground state, is the state in which the electron revolves in the orbit of the smallest radius, the Bohr radius, a0.
Ionization energy:
The ionisation energy is the smallest amount of energy needed to liberate an electron from a hydrogen atom’s ground state.
- The first atomic model was proposed by J.J. Thomson in 1898, according to this model the positive charge of the atom was uniformly distributed throughout the volume of the atom and negatively charged electrons are embedded in it like seeds in watermelon it play an important role in atoms class 12 notes.
Rutherford’s nuclear model: Entire positive charge and most of the mass of an atom are concentrated in a small volume called the nucleus, with electrons revolving around the nucleus just as planets revolve around the sun. This model could not explain(i) Stability of atom,(ii) The line spectra of atoms it play an important role in atoms class 12 notes.
- Bohr’s model of hydrogen atom: Bohr proposed a model for hydrogen and hydrogen-like atom, which explained the line spectra emitted by atoms play an important role in atoms class 12 notes, according to this model only those orbits are stable for which angular momentum of the electron is an integral multiple of h/2π it play an important role in atoms class 12 notes.
- de-Broglie’s explanation to Bohr’s postulate of quantization: He assumed electrons to be particle waves, therefore, the circumference of the stable orbit should be an integral multiple of the wavelength of the wave associated with the electron it play an important role in atoms class 12 notes.
Course Content
Class-12 Physics Ch-12 Atom’s
Lecture-1 (Introduction, J.J Thomson’s Experiment, Rutherford Experiments)
00:00Lecture-2 (Distance of Closest Approach, Energy of Orbiting Electrons, K.E, P.E and T.E)
00:00Lecture-3 (Niels Bohr Model of Atom’s, Bohr First, Second and Third Postulate)
00:00Lecture-4 (Emission Spectrum for Hydrogen Atom’s, Lyman, Balmer, Pashcen, Brackett, Pfund Series)
00:00Lecture-5 (Bhautik Study Book MCQ, Chap Gye Question important for examination point of view)
00:00Lecture-6 (Limitation of Bohr Model, Bhautik Study Book’s Numerical, FAQ)
00:00Lecture-6 (Limitation of Bohr Model, Bhautik Study Book’s Numerical, FAQ)
00:00
Student Ratings & Reviews